What Is Water Molecule Best Described as
This imparts partial negative charge to the oxygen atom and partial positive charge to hydrogen atoms. A mingling of molecules andor ions.
Water Structure Properties Molecule Physical Properties A Level
3 water is one of the many hydrophobic molecules.

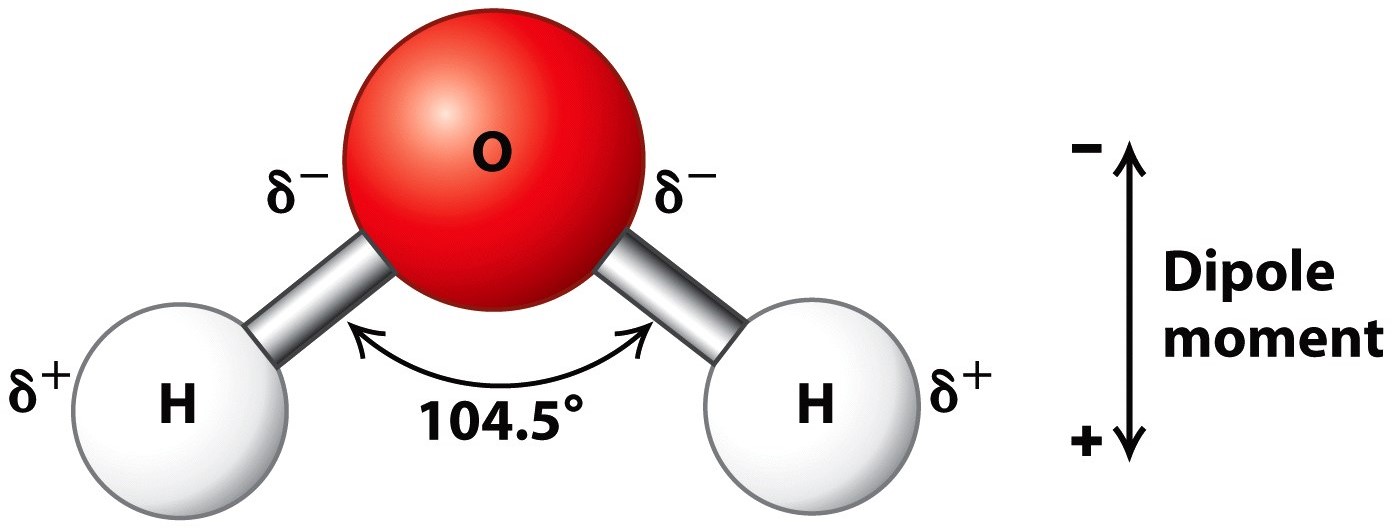
. Air is composed of nitrogen. The oxygen atom is flanked on either side by two hydrogen atoms at an angle of about 10445 degrees. H2O or water as it is more commonly known as is a molecule consisting of 2 Hydrogen molecules bonded to one Oxygen molecule.
Relative to other substances water tends to resist changes in temperature. When describing the size of a water molecule be aware that the shape of an individual water molecule is not a perfect sphere. This means that _____.
There are millions of these molecules in one drop of water. Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic. Water is a polar molecule and also acts as a polar solvent.
Yes it is not a. Thus we can conclude that the statement an oxygen atom shares an electron pair. Water H2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid nearly colorless with a hint of blueThis simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the universal solvent for its ability to dissolve many substances.
The water molecule is neutral but it does exist in equilibrium with hydrogen cation and hydroxide anion that are resp. Dissolving is best described as. Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds.
Water H 2 O essentially considered one of the most important substances found on the earth. A change from a solid to a liquid. The unequal sharing of electrons within a water molecule makes the water molecule _____.
So for every 10 million water molecules there is 1 hydeogen cation and one hydroxide anion. In this molecule what type of bond is found between the oxygen and hydrogens. The water molecule is very simple.
4 the atoms in water. Well electronegativity is the measure of how attracted bond seeking electrons are to an element. Select the statement that best describes a buffer.
2 water molecules are linear like a pole. Hydrophilic What is the name of the spherical particles formed in solution when specks of oil or grease are surrounded by soap molecules. 1 the opposite ends of the molecule have opposite electrical charges.
Molecules breaking into ions. Which best describes how charges are distributed on a water molecule. A water molecule consists of one oxygen atom bonded to two hydrogen atoms by covalent bonds.
That is it has one side that is positively charged and one side that is negatively charged. Therefore an oxygen atom combines with two hydrogen atoms by sharing of electrons. Water is a polar molecule.
Since oxygen is more electronegative as compared to hydrogen atoms the shared electrons are attracted towards the oxygen atom. It is called H2O because it has two atoms of hydrogen H and one atom of oxygen O. It covers over 70 of the earths surface and makes up as much as 95 of the living organisms.
As the table shows this makes H2O a molecule with a polar covalent bond. A separation of molecules into neutral atoms. The chemical formula for water is H 2 O which means each molecule of water consists of one oxygen atom chemically bonded to.
When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. What best describes the bonding in a water molecule points An oxygen atom shares an electron pair with An oxygen atom shares a single electron with each H An oxygen atom receives an electron from each Hatom. The Oddly-Shaped Water Molecule.
A water molecule gained an hydrogen ion from another water molecule. The scientific name for water is H2O. The DNA molecule is best described as a double helix.
Whereas a bond formed by transfer of electrons is known as an ionic bond. A water molecule because of its shape is a polar molecule. The tendency of an atom to pull electrons toward itself is referred to as its _____.
A molecule is the combination of two or more atoms which may exist free in nature. Atoms are the tiniest pieces of matter. Themolecule is made up of two hydrogen atoms and one oxygen atom.
Charged 1 and -1 with an equilibrium constant of 10e-14. This allows it to be the solvent of life. The most common example of this is water.
The molecule that is best described as glucose has the chemical formula C6H12O6. A molecule is a piece of matter that contains two or more atoms. For example hydrogen has 1 electron in its valence shell and oxygen needs 2 electrons to completely fill its orbital.
A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. What is the diatomic nitrogen molecule best described as. A molecule is best described as.
The carboxylate head of the soap molecule is best described as which of the following. The bonds between the atoms are called covalent bonds because the atoms share electrons. Air is composed of nitrogen oxygen argon carbon dioxide and other gasses How may air be best described.
Indeed water as found in nature. The oxygen end is negative relative to the end with the two hydrogen atoms. Structure of water molecule is made up of one molecule of oxygen and two molecules of hydrogen bonded covalently.
Does this mean that one atom of heliom is NOT a molecule. Water as a Compound and Molecule A compound forms whenever two or more atoms form chemical bonds with each other. The positive charge comes from the atomic nucleus while the electrons supply the negative charge.
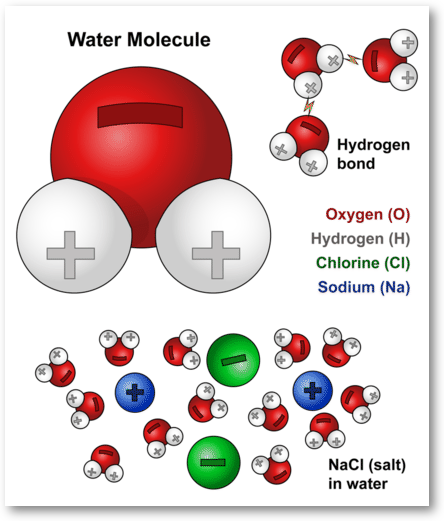
Water Molecules And Their Interaction With Salt U S Geological Survey

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

0 Response to "What Is Water Molecule Best Described as"
Post a Comment